MI Profile
MI Profile
MI Profile® comprehensive profiling assesses DNA, RNA and Proteins from tumor tissue to reveal a more complete molecular blueprint that helps guide precise and individualized treatment decisions. Tests include next-generation sequencing (NGS), immunohistochemistry (IHC), chromogenic in situ hybridization (CISH), pyrosequencing, and AI-predictive algorithms for select tumor types.
- NGS: MI Cancer Seek® (CDx) is performed when ordering MI Profile. If the sample does not meet specimen requirements, the order will reflex to MI Tumor Seek Hybrid™ (LDT).
- MI Cancer Seek (CDx)
- MI Tumor Seek Hybrid (LDT)
- IHC: 25+ tumor relevant protein biomarkers
- CISH: EBER (Gastric/GEJ and Head & Neck) and HPV (Head & Neck)
- Pyrosequencing: MGMT Methylation (Glioma)
- AI Signatures: Caris GPSai® for CUP cases and Caris FOLFIRSTai® for mCRC cases
SPECIMEN TYPE(S)
Tissue (FFPE)
Tissue (Fresh)
APPLICATION
Profiling for therapy selection
DOCUMENT DOWNLOADS
Comprehensive Molecular Profiling

TECHNOLOGY
Next-generation sequencing (NGS), IHC, CISH, PyroSeq
APPLICATION
Biomarker Analysis for Therapy Selection
SPECIMEN TYPE
Tissue
Next generation sequencing
Whole exome
Whole transcriptome
MOLECULAR AI
Caris FOLFIRSTai (mCRC cases)
Caris GPSai (CUP cases)
PROTEIN (IHC) COVERAGE
ALK
AR
CLDN18
ER
FOLR1
Her2/Neu
MAGE-A4
MET
MMR (MLH1, MSH2, MSH6, PMS2)
p16
PD-L1 (22c3, 28-8, SP-142, SP-263)
PR
PTEN
Actionable Insights from Tissue
MI Profile comprehensive testing delivers whole exome sequencing (WES – DNA) and whole transcriptome sequencing (WTS – RNA) of 23,000+ genes, as well as protein analysis and predictive AI algorithms. The test is designed to reveal a more complete molecular blueprint that can guide precise and individualized treatment decisions to help improve patient outcomes.

Immunohistochemistry
(IHC)
25+ tumor expressed proteins
Order Profiling
Email the completed form(s) to CustomerSupport@CarisLS.com, or fax to 1.866.479.4925. When specimen is being prepared for shipment, please include completed forms with the shipper.
For international orders, email the completed
form to InternationalSupport@CarisLS.com, or
fax to 00 41 21 533 53 01.
*Excluding EEA, EU, CH countries.
Tour Our Tissue Lab
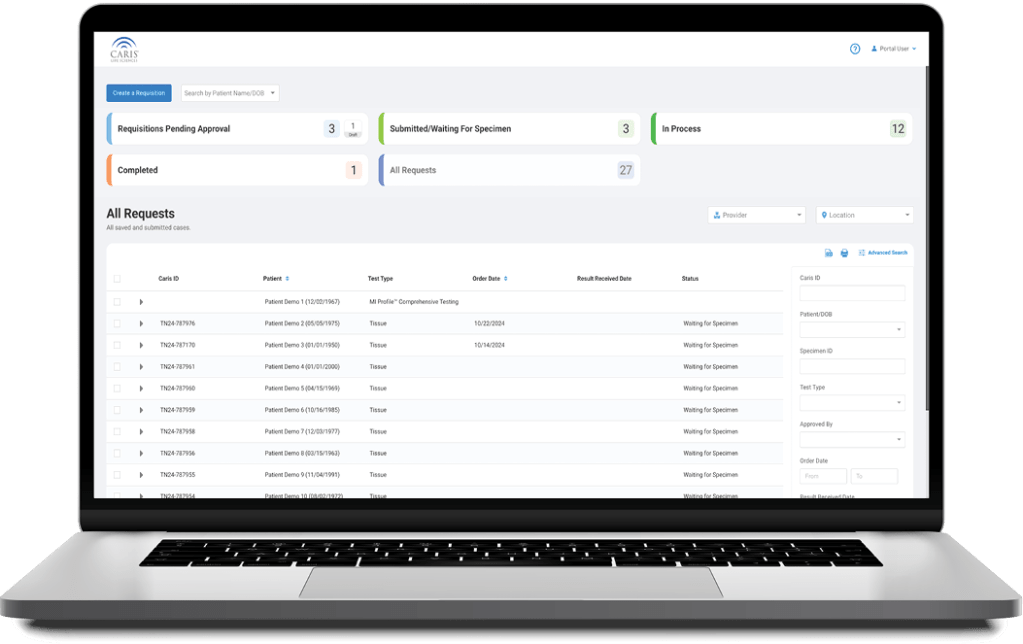
Caris+Portal
Convenient Access to Caris Profiling
Caris+™Portal provides easy access for users to electronically submit orders, track case progress, view results and review Caris Life Sciences’ profiling information in one convenient location.
New users will select the Register link at the bottom of the login page and enter their name and their clinic or institution email address to verify the account.

Complete Molecular Intelligence® Report
The Caris Molecular Profiling Report delivers high impact results, including potentially relevant, actionable clinical information, in an easy-to-interpret format. Every report includes access to the MI Portal and the Clinical Trials Connector™, which matches each patient’s unique biomarker expression profile to open, pertinent clinical trial opportunities.
| Technical Info | IHC | CISH |
|---|---|---|
| Sample Requirements
(see requisition for full details) | 1 unstained slide at 4μm thickness from FFPE block, with evaluable tumor present, per IHC test | 1 unstained slide at 4μm thickness from FFPE block, with at least 100 evaluable tumor cells present, per CISH test |
| Sensitivity/Specificity | >95% | >95% |
Discover
More
Whole Exome and Whole Transcriptome Sequencing from Blood. This revolutionary, pan-cancer assay utilizes a novel circulating Nucleic Acid Sequencing (cNAS) approach. With deep molecular insights from a minimally invasive blood sample, Caris Assure® delivers uncompromising reliability and performance to inform personalized treatment decisions and help improve patient outcomes.
Caris has amassed an industry leading database of molecular and clinical data with more than 660,000 matched patient records. This data enables novel molecular signatures to help improve patient outcomes.