Caris FOLFIRSTai
Navigate mCRC Standard-of-Care Chemotherapy with Caris FOLFIRSTai
Caris FOLFIRSTai® is the first clinically validated, AI-powered molecular predictor of chemotherapy efficacy for mCRC patients, and is included for Caris molecular profiling mCRC cases at no additional cost, increased turnaround time or added specimen requirements.
Caris FOLFIRSTai Validation Data
Traditionally, FOLFOX or FOLFIRI chemotherapy (in combination with bevacizumab) are considered for first-line metastatic colorectal cancer (mCRC) treatment, when patients are unable to tolerate FOLFOXIRI. Caris offers oncologists an Artificial Intelligence (AI)-based predictor intended to gauge a mCRC patient’s likelihood of benefit from first-line FOLFOX+BV followed by FOLFIRI+BV compared to benefits from first-line FOLFIRI+BV followed by FOLFOX+BV treatment.
Comprised of 5,000 machine learning algorithms, Caris FOLFIRSTai identifies the treatment which offers the best benefit as a first-line option for each patient based on the patient’s molecular profile. During validation with two independent data sets (real-world evidence and Phase III TRIBE2 study data), Caris FOLFIRSTai demonstrated that the overall survival (OS) of patients treated in a manner consistent with the FOLFIRSTai prediction was 17.5 months longer (71%) than the OS of patients treated counter to the prediction.1
- 467 cases were manually curated cases with real-world evidence (RWE), which includes data acquired from insurance claims records, electronic medical records and death registries.
- 296 cases analyzed retrospectively from the randomized, prospective phase III TRIBE2 study.
| Median Overall Survival | Caris FOLFIRSTai Indicates: | |
|---|---|---|
| FOLFOX+BV 1st→FOLFIRI+BV 2nd (FOLFOX+BV RWE cohort) |
FOLFIRI+BV 1st→FOLFOX+BV 2nd (FOLFIRI+BV RWE cohort) |
|
| OS When Patient Received: FOLFOX+BV 1st→FOLFIRI+BV 2nd (Figure A) |
42.0 Months | 18.7 Months |
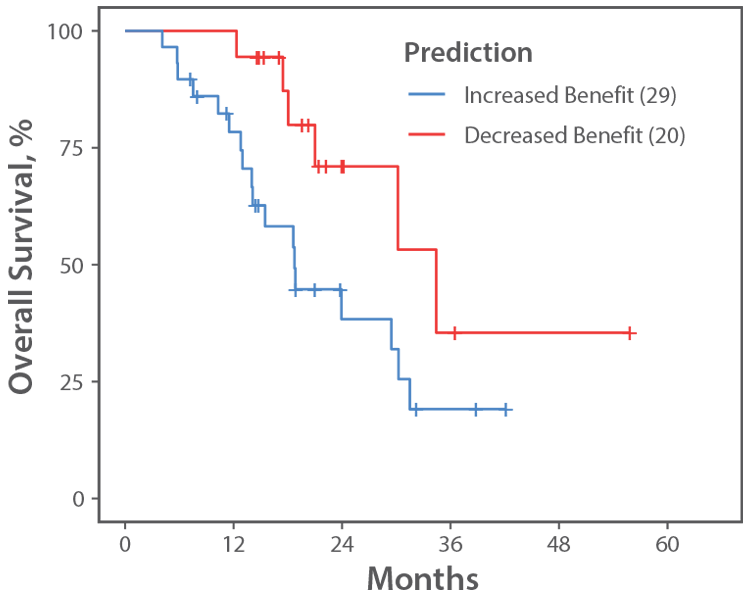
| OS When Patient Received: FOLFIRI+BV 1st→FOLFOX+BV 2nd (Figure B) |
24.5 Months | 34.4 Months |
Figure A.
mCRC RWE FOLFOX+BV−FOLFIRI+BV
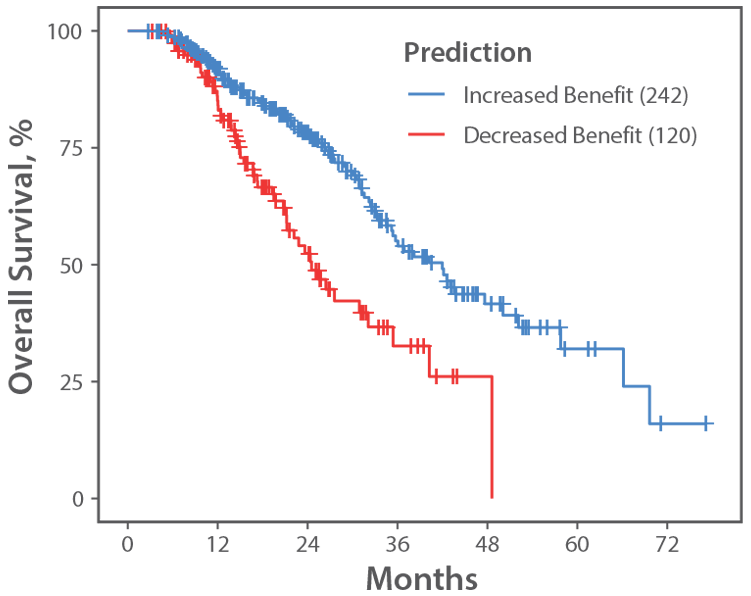
Figure B.
mCRC RWE FOLFIRI+BV−FOLFOX+BV
How it Works
Comprehensive tumor profiling with molecular profiling is performed – Caris FOLFIRSTai is included for Caris molecular profiling mCRC orders. State-of-the-art AI is applied to the molecular testing results to identify a unique molecular signature that predicts a mCRC patient’s likelihood of benefit from first-line FOLFOX+BV followed by FOLFIRI+BV, or FOLFIRI+BV followed by FOLFOX+BV treatment.
mCRC Patients Eligible for First-Line FOLFOX or FOLFIRI Treatment
Consider FOLFOX+BV First, Followed by FOLFIRI+BV

Consider FOLFIRI+BV First, Followed by FOLFOX+BV

Read the Caris FOLFIRSTai Publication
FOLFOX, FOLFIRI, or FOLFOXIRI chemotherapy with bevacizumab is considered standard first-line treatment option for patients with metastatic colorectal cancer (mCRC). We developed and validated a molecular signature predictive of efficacy of oxaliplatin-based chemotherapy combined with bevacizumab in patients with mCRC.
- NCCN Clinical Practice Guidelines in Oncology, Colon Cancer. Version 5.2024.
- Cervantes, A. et al., FOLFOXIRI plus bevacizumab as standard of care for first-line treatment in patients with advanced colon cancer. ESMO Open, Volume 8, Issue 2, 100883
Discover
More
Caris CODEai™ is the most comprehensive, real-world data platform integrating Caris’ extensive catalog of molecular data with cancer treatment information and clinical outcomes data for more than 484,000 patients covering greater than one million data points per patient.
Caris GPSai®, a Genomic Probability Score, uses whole exome (DNA) sequencing and whole transcriptome (RNA) sequencing coupled with machine learning to aid in identifying the tissue of origin.
