Incidental Clonal Hematopoiesis
Clonal Hematopoiesis Identification is Vital for Therapy Selection
Clonal Hematopoiesis (CH) mutations can complicate interpretation of liquid biopsies. When these non-tumor variants occur in genes that are biomarkers for therapy selection in solid tumors, plasma-only liquid biopsy assays can misidentify them as tumor-derived, potentially leading to ineffective and costly off-target treatment.1
Caris Assure Plasma + Buffy Coat Sequencing
Caris Assure™ is a blood-based liquid biopsy assay that analyzes circulating, cell-free DNA and RNA from plasma (cfDNA or cfRNA), plus genomic DNA and messenger RNA from circulating white blood cells (WBCs). By sequencing nucleic acid material from both the plasma and buffy coat (WBC) layers of whole blood, Caris Assure is able to distinguish the tumor-derived variants from incidental sources that often interfere with plasma-only profiling assays. This plasma plus buffy coat sequencing helps to minimize false positives and enhance assay specificity.2
Clonal hematopoiesis mutations are somatic alterations that accumulate in the blood with age and can lead to false positives in liquid biopsy tests, complicating diagnosis and treatment decisions.
Clonal Hematopoiesis and Aging

Clonal hematopoiesis mutations from aging white blood cells may be present in plasma as cfDNA or cfRNA, causing clinical false positive results when analyzing plasma for somatic tumor therapy selection.
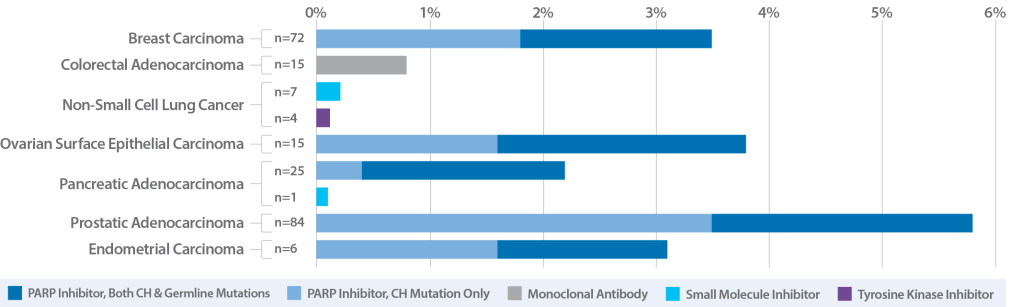
Plasma-only assays that analyze cell-free DNA (cfDNA) cannot distinguish tumor-derived mutations from non-tumor CH variants circulating in the plasma, leading to clinical false positives, interpretation error and selection of inappropriate therapies. For example, about 10% of prostate cancer patients exhibit CH interference in the DNA repair genes used to determine PARP inhibitor (PARPi) eligibility.3 If a PARPi therapy (e.g., olaparib) is selected based on this false positive result, the likely outcomes will include patient side effects, costly but ineffective treatment, and lost time while the tumor continues to grow.
The College of American Pathologists (CAP) and Association for Molecular Pathology (AMP) recommend that cfDNA assays should incorporate whole blood controls to differentiate CH from tumor-derived variants.4
Treat the Tumor, Not the False Positives
In a study of 16,812 patients with advanced cancer across 49 tumor types, Caris Assure identified more than 40% of patients as having at least one clonal hematopoiesis variant among reportable clinical genes.5 By correcting for these non-tumor variants, Caris Assure is able to improve assay specificity and overall performance, providing confidence to physicians making therapy decisions based on profiling results.
Prevalence of Clonal Hematopoiesis by Gene
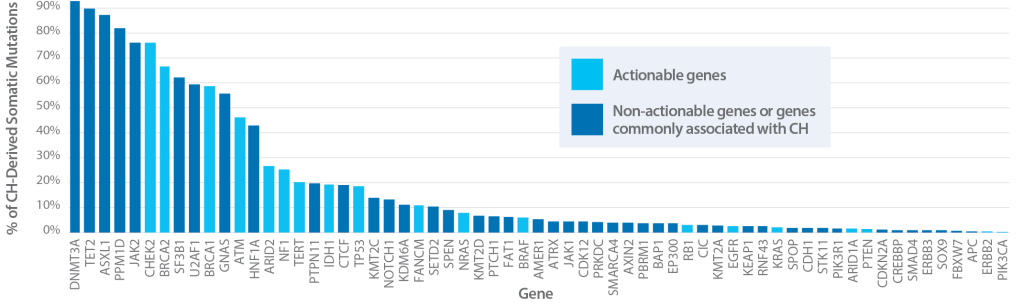
42.3% of 16,812 patients had clonal hematopoiesis mutations in reportable clinical genes including BRCA2, BRCA1, KRAS, BRAF, ATM, and CHEK2.
If only plasma is analyzed, these mutations could be interpreted as tumor-derived, leading to improper therapy selection.
Plasma Plus Buffy Coat is Superior to Plasma-only Sequencing
Caris Assure™ is the only liquid biopsy that analyzes both plasma and buffy coat variants, providing a more complete molecular profile. Distinguishing the source of each variant results in high sensitivity balanced with high specificity, improving overall accuracy to help guide therapy decisions.
Plasma-only liquid biopsies leave unanswered questions.
Plasma contains a mixture of cell-free variants derived from tumor, blood and other cells. These variants cannot be separated based on plasma sequencing alone.1
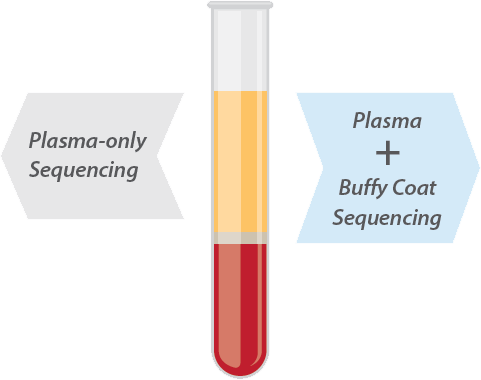
Caris Assure provides the answers needed for therapy selection.
By sequencing plasma and buffy coat (white blood cells) separately, Caris Assure identifies not only somatic tumor mutations but also mutations present in both layers as incidental germline* or clonal hematopoiesis (CH), reporting tumor variants more accurately.
*Not a replacement for comprehensive germline testing. Incidental pathogenic alterations are reported, including ACMG recognized cancer genes. Negative results do not imply the patient does not harbor a germline mutation.
Direct Sequencing of White Blood Cells is Superior to Bioinformatic Prediction
Joint consensus CAP/AMP 2023 recommendations include performing “matched white blood cell [ie, buffy coat] sequencing.”4
Plasma-only assays do not provide enough sequencing information to confirm the source of plasma variants, introducing interpretation error and patient therapy risks.

Discover
More
Caris Assure is a minimally invasive blood test that utilizes circulating Nucleic Acid Sequencing (cNAS) to analyze cell-free DNA and RNA from plasma, plus genomic DNA and messenger RNA from circulating white blood cells (WBCs), to distinguish somatic tumor, incidental clonal hematopoiesis and incidental germline variants.
The results of this large study highlight the need for comprehensive CH classification during liquid biopsy to appropriately recommend therapies, especially PARP inhibitors. Clinicians should avoid reliance on blood profiling results for therapy selection unless the assay utilizes whole blood controls to specifically identify and exclude false positives due to confounding CH variants. Caris Assure is one such assay.
Have Questions?
"*" indicates required fields
References
- Abbosh, C., et al. (2019). Clonal haematopoiesis: a source of biological noise in cell-free DNA analyses. Ann Oncol 30, 358-359
- Abraham, J, Domenyuk, V, et al. (2024) Poster presented at AACR 2024; manuscript submitted.
- Jensen, K, et al. (2021) JAMA Oncol. 7, 107–110.
- Lockwood CM, et al. (2023) J Mol Diagn 25, 876-897.
- Magee, D, Domenyuk, V, Abraham, J, et al. (2025) Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-24-3335