Caris Precision Oncology Alliance
Advancing Science, Shaping the Future of Precision Medicine
The Caris Precision Oncology Alliance™ (POA) is a leading global cancer research enterprise, exclusively focused on precision oncology to optimize clinical care and outcomes for cancer patients. The Caris POA is a best-in-class collaborative research network of leading physicians and oncologists to identify predictive and prognostic markers to further advance the integration of molecular profiling into all aspects of cancer care.

“At Caris, we have access to unprecedented tools for understanding tumor biology, which we leverage to support front-line clinicians in delivering better patient outcomes. Through the Precision Oncology Alliance (POA), we empower researchers with access to powerful data sets, enabling them to ask and answer the critical questions that are advancing the field of precision medicine.”
-James Hamrick, MD, MPH, Chairman, Caris Precision Oncology Alliance
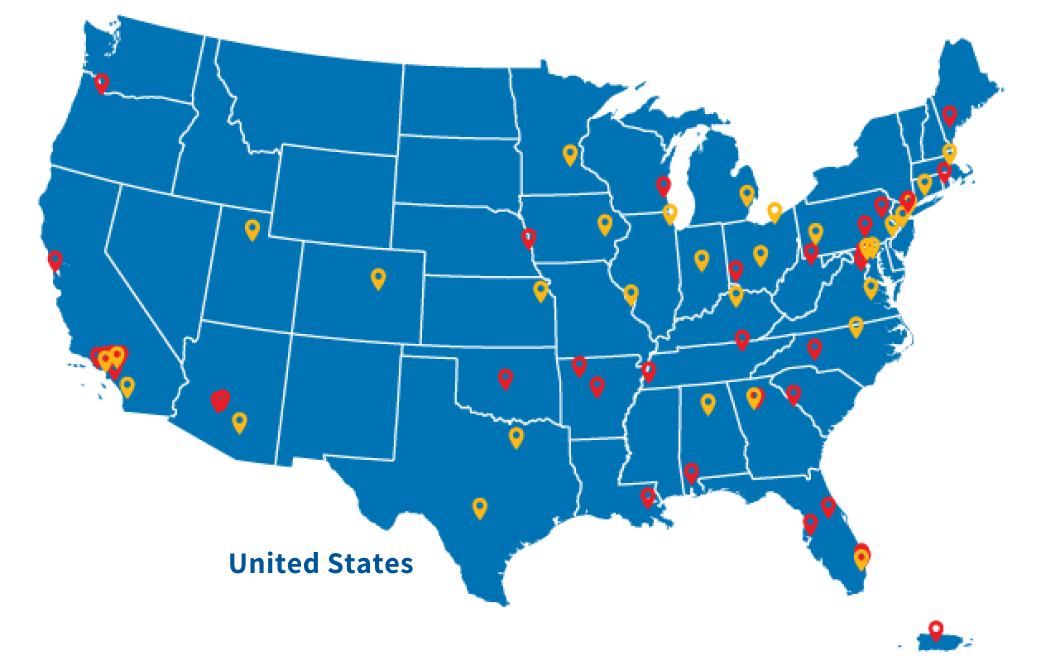
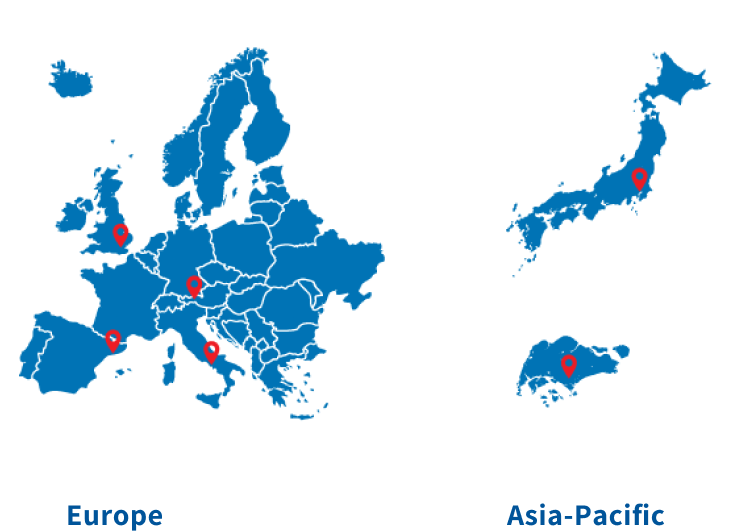
Caris POA Members & Locations
The Caris POA includes more than 95 leading cancer institutions and research consortia, including over 45 NCI-designated comprehensive cancer centers, collaborating to deliver molecular science solutions that promote clinical trials, deliver combined clinical-genomic data, and advance collaborative research and publications.

POA SITE

NCI-DESIGNATED POA SITE
Caris POA Leadership
The Caris POA Executive Committee establishes appropriate molecular testing principles to serve as guidelines for the consistent application of tumor profiling across the oncology community. Responsible for facilitating collaborative research studies across the Caris POA and coordinating outcome tracking initiatives, the Committee helps to generate and document additional medical evidence for the appropriate use and impact of profiling.

James Hamrick, MD, MPH
Chairman, Caris Precision Oncology Alliance

Manmeet Ahluwalia, MD, MBA
Chief, Solid Tumor Medical Oncology Deputy Director, Chief Scientific Officer Miami Cancer Institute

Heinz-Josef Lenz, MD, FACP
Associate Director for Clinical Research USC Norris Comprehensive Cancer Center

Sagar Lonial, MD, FACP
Chief Medical Officer Professor and Chair Emory University

Marcia Cruz-Correa, MD, PhD
Executive Director University of Puerto Rico

Ruben Mesa, MD
President / Executive Director Atrium Health Levine Cancer / Wake Forest

John L. Marshall, MD
Chief, Hematology and Oncology Georgetown University

Thomas Herzog, MD
Professor of OB-GYN Deputy Director University of Cincinnati Cancer Institute

Wafik El-Deiry, MD, PhD, FACP
Associate Dean for Oncologic Sciences Brown University
Working Groups
The Caris POA has working groups across various oncology specialties. Caris POA members are invited and encouraged to participate in these groups based upon research interest. The groups meet regularly, focusing on research strategies, publication activities and protocol development within the disease state of interest. Participating in these virtual meetings allows for improved collaboration, increased publication productivity and better peer exchange.
- Breast
- Central Nervous System (CNS)
- Cutaneous
- Gastrointestinal (GI)
- Genitourinary (GU)
- Gynecologic Malignancies (GYN)
- Head & Neck
- Neuroendocrine (NET)
- Pathology
- Pediatrics/AYA
- Sarcoma
- Thoracic
- Translational
Resources that Empower POA Members
At the forefront of molecular science and artificial intelligence, Caris Life Sciences is deeply committed to bringing the vision of precision medicine to life. Caris achieves this by delivering advanced diagnostic testing and crucial molecular insights, enabling POA partners to enhance cancer care and improve patient outcomes. With a distinctive blend of expertise, proprietary technologies, and a vast data repository, Caris collaborates with the POA, charting innovative courses in precision medicine.

Caris CODEai
Delivers value through a growing database with over 484,000 clinical outcomes paired with matched molecular data.

Research Initiation (Letter of Intent Process)
Engaging with Caris often involves proposing and initiating collaborative research projects within the POA membership for advancing research efforts.

Molecular Minute Podcast Series
Gain valuable insights from today’s foremost oncology experts on a wide array of leading-edge subjects in precision oncology and the evolution of cancer care.

Publications
The POA continues to amplify the significance of biomarker analysis and tumor profiling in cancer care, fostering a deeper understanding of molecular science and propelling the evolution of precision medicine through its publications.

Caris Molecular Tumor Board
Facilitates collaboration between clinicians and top oncologists to analyze complex molecular data, providing tailored therapeutic recommendations and support for challenging cancer cases.

Right-In-Time Trials
Caris’ Right-In-Time clinical trial network eliminates repetitive contracting, feasibility and site qualification activities so providers can focus on treating their patients, rather than the administrative burden of clinical trial start-up.
Discover
More
Meet the leadership team working to deliver on the promise of precision medicine.
Read the latest news and press releases to stay up to date with the innovative work of Caris.
Ready to Get Started on the Journey?
"*" indicates required fields